
NewsInformation Center
Why do you use a flammability test for polymer composites?
2023/02/07
Polymer composites, as a common ignition material in modern fires, especially in building fires and urban fires, have a special combustion behaviour and the changes in thermal behaviour, pyrolytic chemical processes and combustion processes in fires are very complex. polymer composites often show some special behavioural changes in fires, such as melting, foaming, expansion They have a certain influence on the pyrolysis process, ignition and combustion processes. In addition, the course of the pyrolysis reaction mechanism of polymer composites may also be altered by the action of high heat flow in fires. Despite a great deal of research into fires by scholars at home and abroad, the efforts of polymer composites to achieve fire prevention through flame retardant treatment still face a great challenge due to the complexity of the problem and the interlinked interactions of chemical processes, mass and heat transfer.

Why do you use a flammability test for polymer composites?
The flame retardancy of polymer composites needs to be measured because the foamed materials of polymer composites have the characteristics of light weight, low thermal conductivity, and easy processing. Therefore, it is necessary to measure its flame retardant performance during use.
Polymer composites generally refer to polymer compounds, also known as macromolecules, which are compounds with a relative molecular mass of several thousand to several million. Most polymer compounds are mixtures of many homologues with different relative molecular masses. Therefore, the polymer composites The relative molecular mass is the average relative molecular weight.
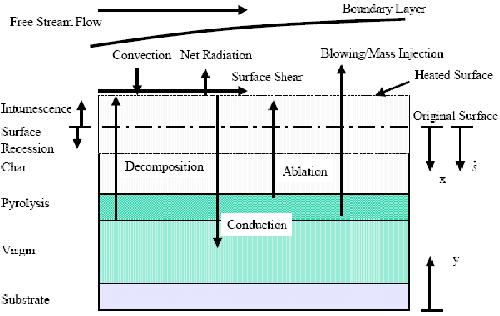
Pyrolysis and Combustion Processes of Polymer Materials
The combustion process of polymers not only has the basic characteristics of the combustion of general combustible solid materials, but also has some distinctive characteristics. These properties are reflected both during heating of the polymer prior to ignition and during ignition and combustion. Phenomena such as softening, melting, expansion, foaming, and shrinkage are special thermal behaviors of many polymers during heating and burning. Figure 1 describes some special physicochemical phenomena that occur during pyrolysis. During the decomposition process, different polymers also undergo reaction processes with different mechanisms, involving random decomposition, depolymerization, cyclization, crosslinking reactions, etc., and produce various decomposition products. What is more complicated is that the mechanism and kinetics of these chemical reactions may also be affected by changes in the external heating environment and changes in the thermal behavior of internal materials. Both these processes and their final decomposition products have an important influence on the combustion process.
Environmental heat is the main external condition leading to polymer combustion. At the same time, combustion is a process of oxidative degradation, in which oxygen plays a very important role. For most polymer degradation, free radical chain reactions, including hydrogen transfer, dehydrogenation, etc., especially in the presence of oxygen, make polymer degradation easier. The thermal oxidative degradation reaction of polymers is generally carried out according to the free radical chain reaction. The whole reaction process mainly includes three basic reactions: chain initiation, chain growth and chain termination. The chain termination includes disproportionation termination and coupling termination.
Previous: What's the note of the air permeability tester?
N e x t : Fabric shrinkage | Washing dimensional change rate test method



